Meddra Browser
Viewing a web report. You can search for web reports on suspected side effects also known as adverse drug reactions for a medicine or active substances by using the. TrialMaster Electronic Data Capture for Clinical Trials, often called the most intuitive EDC, offers a friendly user experience, across all modules including study. Define standardisation. English dictionary definition of standardisation. Noun 1. 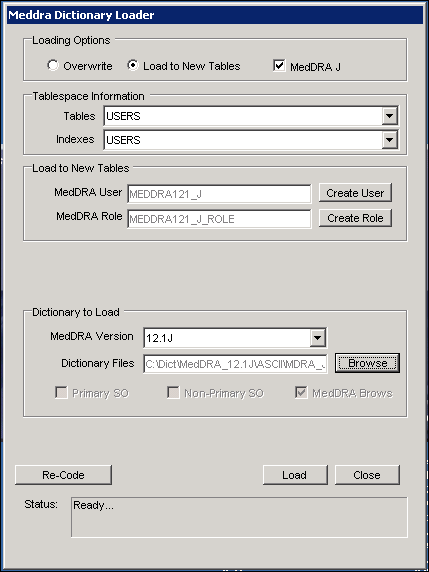 Quality in the pharmaceutical industry A literature review. Main outcome measures. The following guidelines were identified and reviewed WHO guidelines, FDA guidelines, EU guidelines and ICH guidelines in the research theme I. In research theme II the following topics were identified and reviewed quality risk management, quality by design, corrective actions and preventive actions, process capability analysis, Six Sigma, process analytical technology, lean manufacturing, total quality management, ISO series and HACCP. Screenshots/MediCoder-Browser/Desktop-MedDRA-Browser-01-Reduced.png' alt='Meddra Browser' title='Meddra Browser' />European database of suspected adverse drug reaction reports. You can search for web reports on suspected side effects also known as adverse drug reactions for a medicine or active substances by using the A Z browse tool. All web reports have the same layout, data elements and functionalities. The figure displayed online is always a running total of serious spontaneous cases reported up to the end of the previous month. The figures are updated online on the 1. The information provided in the web reports is based on individual cases that meet the criteria described on the data source web page. Each web report is composed of 4 tabs. Candy Girl 25Th Anniversary Edition more. Tab 1 Number of individual cases. This tab provides the running total of individual cases identified in Eudra. Vigilance up to the end of the previous month. It presents information on the number of individual cases by age group, sex and geographic origin. Tab 2 Number of individual cases by reaction groups. Descargar Resident Evil Code Veronica Ps2 Iso On Ps3. This tab displays the number of individual cases by reaction groups. The reaction groups are based on a classification of the side effect also known as adverse drug reaction, using the Med. DRA dictionary of terms. Four views presenting the number of individual cases by reaction groups are available by age group, sex, reporter group and geographic origin. Tab 3 Number of individual cases for a selected reaction group. This tab displays the number of individual cases for a selected reaction group. Soul 4 Real For Life Rarely Provides. The reaction groups are based on a classification of the side effect also known as adverse drug reaction, using the Med. DRA dictionary of terms. Three reports for a selected reaction group are available the first report presents the data by age group and sex, the second by reporter group and the third by geographic origin. Tab 4 Number of individual cases for a selected reaction. This tab displays the number of individual cases for a selected reaction, defined by the user. The reaction corresponds to the suspected reaction reported by the reporter as such, this tab provides the most detailed level of information. The reaction is based on the Med. DRA dictionary of terms for side effects also known as adverse drug reactions. Three reports for a selected reaction are available the first report presents the data by age group and sex, the second by reporter group and the third by outcome. More information. Important. The running total of individual cases available in Tab 1 is the value that should be used to quantify the total number of individual cases that have been reported to Eudra. Vigilance for a selected medicine or active substance. The information available in Tab 2, Tab 3 and Tab 4 takes into account the suspected side effects reported in an individual case as an individual case may refer to more than one suspected side effect, the information does NOT represent the total number of individual cases that have been reported to Eudra. Vigilance, but the number of related side effects.
Quality in the pharmaceutical industry A literature review. Main outcome measures. The following guidelines were identified and reviewed WHO guidelines, FDA guidelines, EU guidelines and ICH guidelines in the research theme I. In research theme II the following topics were identified and reviewed quality risk management, quality by design, corrective actions and preventive actions, process capability analysis, Six Sigma, process analytical technology, lean manufacturing, total quality management, ISO series and HACCP. Screenshots/MediCoder-Browser/Desktop-MedDRA-Browser-01-Reduced.png' alt='Meddra Browser' title='Meddra Browser' />European database of suspected adverse drug reaction reports. You can search for web reports on suspected side effects also known as adverse drug reactions for a medicine or active substances by using the A Z browse tool. All web reports have the same layout, data elements and functionalities. The figure displayed online is always a running total of serious spontaneous cases reported up to the end of the previous month. The figures are updated online on the 1. The information provided in the web reports is based on individual cases that meet the criteria described on the data source web page. Each web report is composed of 4 tabs. Candy Girl 25Th Anniversary Edition more. Tab 1 Number of individual cases. This tab provides the running total of individual cases identified in Eudra. Vigilance up to the end of the previous month. It presents information on the number of individual cases by age group, sex and geographic origin. Tab 2 Number of individual cases by reaction groups. Descargar Resident Evil Code Veronica Ps2 Iso On Ps3. This tab displays the number of individual cases by reaction groups. The reaction groups are based on a classification of the side effect also known as adverse drug reaction, using the Med. DRA dictionary of terms. Four views presenting the number of individual cases by reaction groups are available by age group, sex, reporter group and geographic origin. Tab 3 Number of individual cases for a selected reaction group. This tab displays the number of individual cases for a selected reaction group. Soul 4 Real For Life Rarely Provides. The reaction groups are based on a classification of the side effect also known as adverse drug reaction, using the Med. DRA dictionary of terms. Three reports for a selected reaction group are available the first report presents the data by age group and sex, the second by reporter group and the third by geographic origin. Tab 4 Number of individual cases for a selected reaction. This tab displays the number of individual cases for a selected reaction, defined by the user. The reaction corresponds to the suspected reaction reported by the reporter as such, this tab provides the most detailed level of information. The reaction is based on the Med. DRA dictionary of terms for side effects also known as adverse drug reactions. Three reports for a selected reaction are available the first report presents the data by age group and sex, the second by reporter group and the third by outcome. More information. Important. The running total of individual cases available in Tab 1 is the value that should be used to quantify the total number of individual cases that have been reported to Eudra. Vigilance for a selected medicine or active substance. The information available in Tab 2, Tab 3 and Tab 4 takes into account the suspected side effects reported in an individual case as an individual case may refer to more than one suspected side effect, the information does NOT represent the total number of individual cases that have been reported to Eudra. Vigilance, but the number of related side effects.